
RadioPharmaceutical Therapy (RPT): a major advance in cancer treatment
RadioPharmaceutical Therapy (RPT) represents a significant advance in the fight against cancer. Although this innovative technique currently accounts for only a small proportion of cancer treatments, RPT offers new perspectives for treating malignant tumours by precisely targeting cancer cells while preserving surrounding healthy tissue.
This approach is part of the growing field of theranostics, which aims to improve cancer treatment by combining diagnostic tests that analyse a patient’s tumour activity on an individual basis with targeted therapies tailored to their specific cancer.
What is RPT (RadioPharmaceutical Therapy)?
RadioPharmaceutical Therapy (RPT) is a cancer treatment that involves delivering radionuclides directly inside or next to the tumour, using a specific vector. Unlike conventional external beam radiotherapy, which irradiates the tumour from outside the body, RPT delivers radiation internally, minimising damage to surrounding healthy tissues. RPT is especially effective for treating small tumours and multiple targets like metastases.
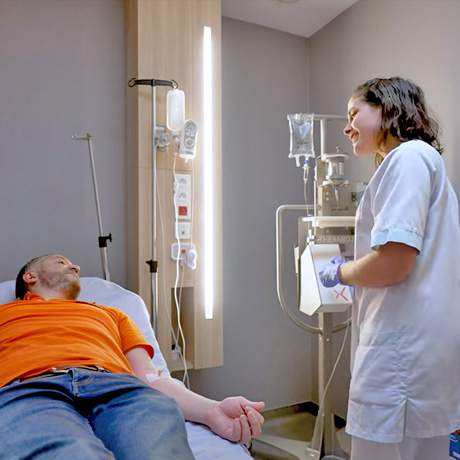
How does RPT (RadioPharmaceutical Therapy) work?
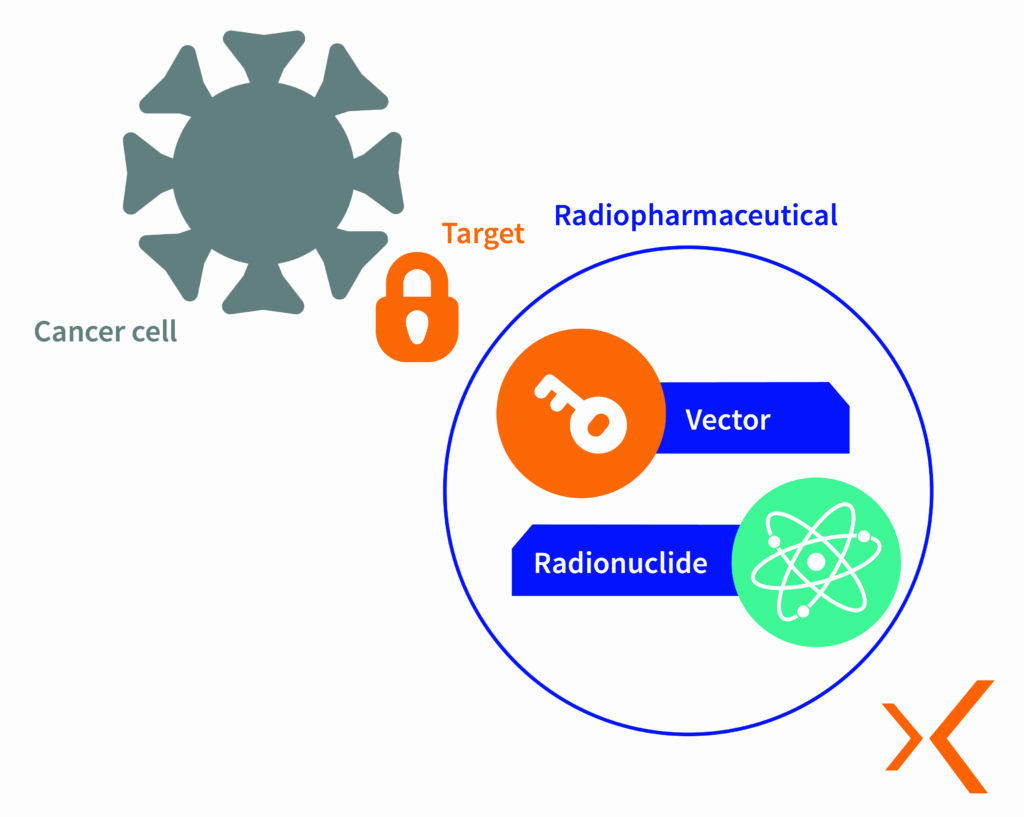
RPT uses specific vectors to selectively target cancer cells. These vectors can be biological molecules such as monoclonal antibodies, peptides or modified viruses. Once administered, these vectors bind to receptors on the surface of cancer cells, allowing the radionuclide to be delivered directly to the target.
The radionuclide, which emits high-energy alpha or beta radiation, damages the DNA of the tumour cells and leads to their destruction. This method delivers a high dose of radiation directly to the tumour while minimising side effects on surrounding healthy tissues.
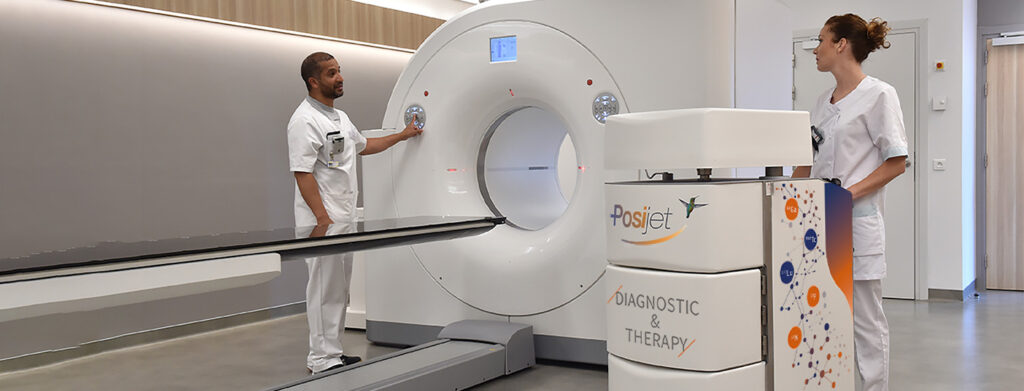
RPT is typically administered intravenously or orally. This theranostic approach aims to “treat what we see and see what we treat” by using preliminary diagnostic imaging to specifically target cancer cells. The imaging technique often uses a β+ emitting radioisotope for Positron Emission Tomography (PET) to visualise the tracer’s distribution in the body. If the patient is eligible for treatment, the radioisotope used for diagnostic imaging is replaced with an α or β– emitter for therapeutic purposes. Once the therapeutic radiopharmaceutical has been administered, a so-called “post-therapeutic” scintigraphy scan can be performed to visualise tracer uptake in target tissues, particularly in the case of γ emissions associated with the therapeutic radionuclide.
RadioPharmaceutical Therapy (RPT) offers several major advantages in oncology:
- Increased efficiency: RPT delivers a higher dose of radiation directly to the tumour, leading to more effective destruction of cancer cells.
- Reduced side effects: By specifically targeting cancer cells, RPT minimises damage to surrounding healthy tissues, thereby reducing the side effects associated with conventional treatments.
- Customised treatment options: RPT uses specific vectors to deliver personalised treatments tailored to each patient and each type of cancer.
- Exploration of new therapeutic targets: RPT opens up new possibilities for targeting therapeutic areas that were previously inaccessible with conventional treatments, providing new options for patients with cancers resistant to traditional therapies.
- Selective patient eligibility: only patients most likely to respond well to this type of therapy are selected.
Successful initial injections
Two of the most commonly used radioisotopes in RPT are iodine-131 and lutetium-177.

Iodine-131 is used in the treatment of hyperthyroidism and differentiated thyroid cancer. This isotope emits β– radiation, which allows a high dose of radiation to be delivered directly to the tumour. Iodine-131 treatment is administered orally and can be done on an outpatient basis for hyperthyroidism, while thyroid cancer patients require a short hospital stay. Unbound iodine is primarily eliminated through urine, and side effects are rare due to its specificity for thyroid cells.

Lutetium-177 is used in the treatment of neuroendocrine tumours and metastatic prostate cancer. As this isotope decays, it emits β– radiation, enabling precise radiation delivery to cancer cells. Two radiopharmaceutical drugs using lutetium-177 are currently available in France: 177Lu-DOTATATE for neuroendocrine tumours and 177Lu-PSMA-617 for prostate cancer. These treatments are administered via intravenous injection every six weeks and are generally well tolerated by patients.
However, the slow administration of these treatments requires optimised radiation protection and a safe and convenient injection system for healthcare personnel. Responding to this need, teams from Beaujon AP-HP hospital and Lemer Pax collaborated to develop an ultra-safe solution: the Theranojet®ARA. This innovation improves the administration of new therapeutic radiopharmaceuticals, providing healthcare professionals with safer and more efficient working conditions.
Specifically designed for RadioPharmaceutical Therapy (RPT), the Theranojet®ARA includes a sterile, needle-free transfer device to minimise contamination and needle-stick injuries.
Watch the video to see how this pump shield integrates seamlessly into your Nuclear Medicine department:
Furthermore, by offering a safe and efficient method for administering theranostic products, the Theranojet®ARA is a key solution in advancing RPT and enhancing safety and efficacy standards in cancer treatment.
RPT represents a significant advancement in oncology, garnering increasing attention and engagement from researchers and physicians
Research is ongoing, with a particular focus on promising new radionuclides such as lead-212, actinium-225, zirconium-89, and copper-64.
RadioPharmaceutical Therapy (RPT) holds significant promise in enhancing treatment effectiveness and improving patient quality of life by reducing side effects associated with conventional therapies. Advances in RPT instil hope and pave the way for new therapeutic strategies in the battle against cancer.









